
Rapid detection of Chlamydia & Gonorrhea
Members have exclusive contract pricing through Medline
Fastest molecular CT/NG test for males and females - allows true test and treat
Laboratory performance in 30 minutes
Fully automated, very easy to use by non-lab-trained personnel
Approximately 1-minute hands-on time per patient sample
No interpretation of results required
Reimbursement codes established
Established CPT codes with reimbursement already in place
Billable with established CPT codes Chlamydia Test 87491 Gonorrhea Test 87591 Infectious Agent, Multiple Organisms & 87801

The current testing model for CT/NG is very inefficient. The time between sample collection and treatment can take between 1- 10 days. 1
The binx io enables a test & treat model that may help the patient, provider and community improve access to CT/NG diagnostic testing and care.
What's in it for me?
Why the binx io is important for me. The binx io CT/NG Assay System:
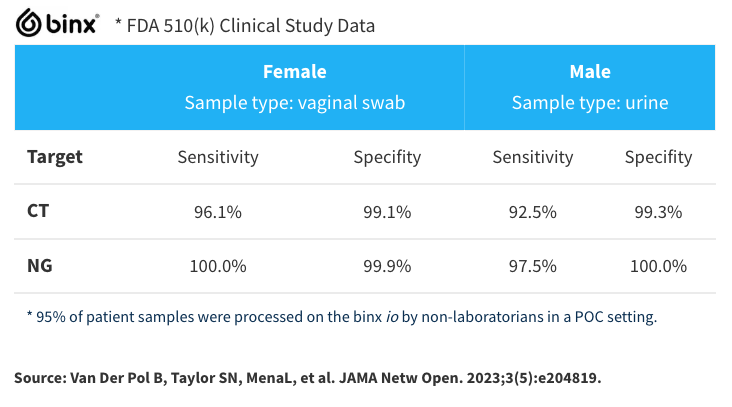
Prospective Clinical study: performance measured against three establish Laboratory platforms






We make it easy to do more for your patients and practice.