Save up to 12% on Vivotif® Oral Typhoid vaccine
Enjoy the lowest prices on all of Emergent's vaccines.

Save up to 6.5% for Vaxchora® vaccine
Enjoy the lowest prices on all of Emergent's vaccines.

Conveniently purchase directly from https://emergenttravelhealth.com
Create a Bavarian Nordic account and select PBG via PracticeWell as the GPO.

Rebates earned on all Vivotif® and Vaxchora® purchases
These generous rebates lower your overall cost.

No minimum purchase necessary for contract discount


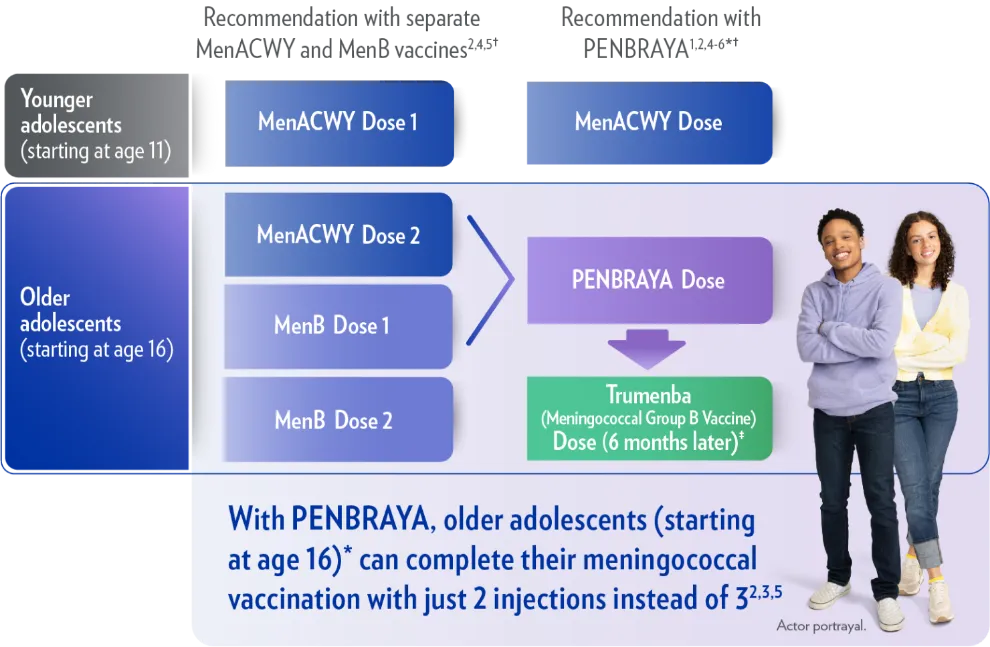
ACIP recommends PENBRAYA for vaccinating your healthy 16-year-old patients against serogroups A, B, C, W, and Y1,2
- The ACIP approved this recommendation by majority vote, and it has been adopted by the CDC Director. It will be published in MMWR in the coming months1
- While PENBRAYA was studied and FDA approved for use as a 2-dose series administered 6 months apart and met all endpoints assessing non-inferiority in clinical trials, the ACIP considers many factors in addition to clinical trial results when deciding how to recommend a vaccine2,3
- According to the ACIP, when the decision has been made to vaccinate against MenABCWY using PENBRAYA, Trumenba must be used to complete the MenB series. B component vaccines are not interchangeable by manufacturer. Administration of a B component vaccine (MenB or MenABCWY) requires that subsequent B component vaccine doses be from the same manufacturer2
ACIP RECOMMENDED1
PENBRAYA may be used when both MenACWY and MenB are indicated at the same visits*
†ACIP recommends routine administration of a MenACWY vaccine for all healthy persons aged 11 to 18 years, with a single dose at age 11 or 12, followed by a booster dose at age 16. ACIP also recommends a 2-dose MenB vaccine series administered 1 to 6 months apart for healthy persons aged 16 to 23 years on the basis of shared clinical decision-making, with a preferred age range of 16 to 18. Visit ACIP Meningococcal Vaccine Recommendations for additional information, including recommendations for individuals at increased risk.1,2
‡PENBRAYA and Trumenba are FDA approved for dosing at 0 and 6 months. There is no data supporting the use of Trumenba at an interval of greater than 6 months.3,6
ACIP=Advisory Committee on Immunization Practices; MMWR=Morbidity and Mortality Weekly Report.
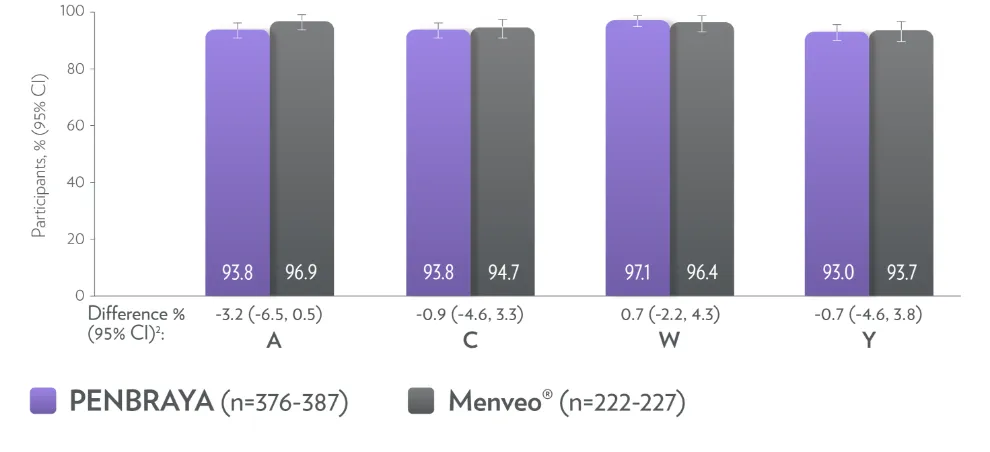
PENBRAYA elicited a robust immune response for serogroups A, B, C, W, and Y 1*†
PENBRAYA demonstrated a similar seroresponse to MenACWY-CRM1,2‡
- According to the ACIP, when the decision has been made to vaccinate against MenABCWY using PENBRAYA, Trumenba must be used to complete the MenB series. B component vaccines are not interchangeable by manufacturer. Administration of a B component vaccine (MenB or MenABCWY) requires that subsequent B component vaccine doses be from the same manufacturer2
PENBRAYA at age 16 provides non-inferior ACWY responses in patients who have previously received ACWY vaccinations
* The LLOQ is an hSBA titer = 1:8 for serogroups A, C, W, and Y. Seroresponse is defined as the 4-fold increase as follows: (1) For participants with a baseline hSBA titer < 1:4 (LOD), a 4-fold response was defined as an hSBA titer ≥1:16. (2) For participants with a baseline hSBA titer ≥LOD and < LLOQ, a response is defined as an hSBA titer ≥4 times the LLOQ. (3) For participants with a baseline hSBA titer ≥LLOQ, a response is defined as an hSBA titer ≥4 times the baseline titer.1
† Evaluable immunogenicity populations.1
‡ Non-inferiority was demonstrated (using 10% margin) post-vaccination by assessing the difference between vaccination groups.1
§ MenACWY-CRM is sold and licensed under the tradename Menveo®. Menveo® is a registered trademark of GSK.3
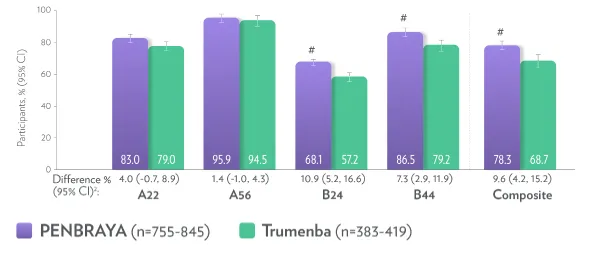
Two doses of PENBRAYA demonstrated similar immunogenic responses to MenB primary strains (representative of US disease-causing strains) compared to two doses of Trumenba (Meningococcal Group B Vaccine)1,2||¶‡‡
In ACWY-exposed individuals, PENBRAYA at age 16 combines the ACWY booster with the first injection of the MenB series. Help more patients start their MenB series.1,4,5
|| The LLOQ is an hSBA titer = 1:16 for A22 and 1:8 for A56, B24, and B44. Seroresponse is defined as the 4-fold increase as follows: (1) For participants with a baseline hSBA titer < 1:4 (LOD), a 4-fold response was defined as an hSBA titer ≥1:16. (2) For participants with a baseline hSBA titer ≥LOD and < LLOQ, a response is defined as an hSBA titer ≥4 times the LLOQ. (3) For participants with a baseline hSBA titer ≥LLOQ, a response is defined as an hSBA titer ≥4 times the baseline titer.1
¶ Evaluable immunogenicity populations.1
‡‡ Non-inferiority was demonstrated (using 10% margin) post-vaccination by assessing the difference between vaccination groups.1
** Composite response = hSBA ≥LLOQ for all 4 primary meningococcal B strains combined.1
†† The MenB vaccine component of PENBRAYA is licensed and sold as Trumenba by Pfizer and is licensed for use in the United States.6
# Statistically higher.1
CI=confidence interval; hSBA=human serum bactericidal assay; LLOQ=lower limit of quantitation; LOD=limit of detection.
Indications
- PENBRAYA is indicated for active immunization to prevent invasive disease caused by Neisseria meningitidis serogroups A, B, C, W, and Y. PENBRAYA is approved for use in individuals 10 through 25 years of age
- TRUMENBA is indicated for active immunization to prevent invasive disease caused by Neisseria meningitidis serogroup B. TRUMENBA is approved for use in individuals 10 through 25 years of age
Important Safety Information
- Do not administer PENBRAYA or TRUMENBA to individuals with a history of severe allergic reaction (eg, anaphylaxis) to any component of PENBRAYA or TRUMENBA. Appropriate medical treatment used to manage allergic reactions must be available in the event an anaphylactic reaction occurs immediately following administration of PENBRAYA or TRUMENBA
- Syncope (fainting) may occur in association with administration of injectable vaccines, including PENBRAYA or TRUMENBA. Procedures should be in place to avoid injury from fainting
- Some individuals with altered immunocompetence may have reduced immune responses to PENBRAYA or TRUMENBA
- Individuals with certain complement deficiencies and individuals receiving treatment that inhibits terminal complement activation are at increased risk for invasive disease caused by N. meningitidis groups A, B, C, W, and Y, even if they develop antibodies following vaccination with PENBRAYA
- Vaccination with PENBRAYA or TRUMENBA may not protect all vaccine recipients
- Guillain-Barré syndrome (GBS) has been reported in temporal relationship following administration of another US-licensed meningococcal quadrivalent polysaccharide conjugate vaccine. The decision by the healthcare professional to administer PENBRAYA to individuals with a history of GBS should take into account the expected benefits and potential risks
- For PENBRAYA, the most commonly reported (≥15%) solicited adverse reactions after Dose 1 and Dose 2, respectively, were pain at the injection site (89% and 84%), fatigue (52% and 48%), headache (47% and 40%), muscle pain (26% and 23%), injection site redness (26% and 23%), injection site swelling (25% and 24%), joint pain (20% and 18%), and chills (20% and 16%)
- For TRUMENBA, the most common solicited adverse reactions in adolescents and young adults were pain at injection site (≥85%), fatigue (≥60%), headache (≥55%), and muscle pain (≥35%)
- Data are not available on the safety and effectiveness of using TRUMENBA and other meningococcal group B vaccines interchangeably to complete the vaccination series
- The safety and effectiveness of PENBRAYA or TRUMENBA have not been established in pregnant individuals